|
Listen to this article  |

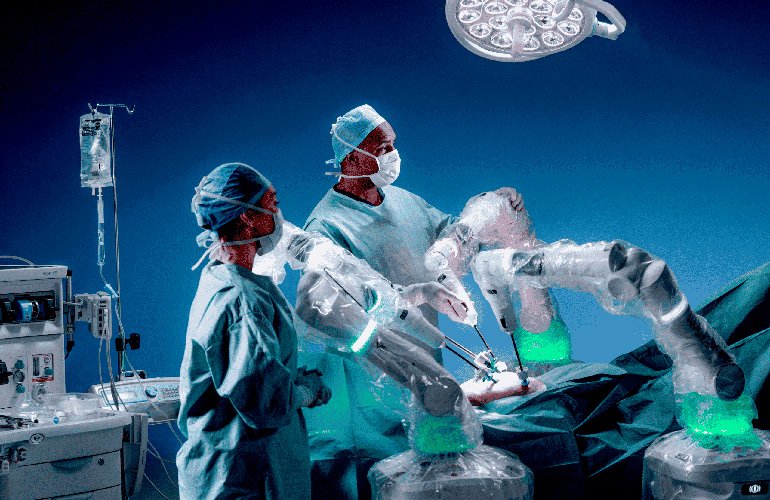
CMR Surgical’s Versius in the operating room with the surgical team.
CMR Surgical has initiated its first multicenter clinical trial to assess the safety and efficacy of its Versius System for use in pediatric surgery. The study focuses on children and infants undergoing a range of urological procedures with Versius. These include robotic-assisted pyeloplasty, ureteroureterostomy, nephrectomy, and mitrofanoff formation, among others.
The trial will be carried out in collaboration with three NHS clinical sites. The primary site of the study is the Department of Pediatric Urology at Southampton Children’s Hospital. This hospital was the first to perform pediatric urological surgery with Versius in a clinical trial.
“It is a privilege to be part of such a fantastic team here at Southampton; there is a huge list of people who helped us get to the point where we were able to start the first clinical trial using Versius in children, and I am truly grateful to all of them,” Ewan Brownlee, principal investigator of the prospective clinical trial and consultant. said a pediatric surgeon at Southampton Children’s Hospital. “It was also a pleasure to work with excellent colleagues in Manchester and Evelina and with CMR to carry out this test. We are excited about what we feel is a breakthrough step forward in the development of pediatric robotic surgery.”
Versius is a small, modular and versatile surgical robot used for robotically assisted inferior access surgery, a form of keyhole surgery. The small and modular design of Versius allows surgeons to access small, hard-to-reach spaces in the operating area while maintaining clear access to the patient for the anesthesiologist and the wider surgical team.
 Register now and save.
Register now and save.
More details about the study
The Department of Pediatric Urology at the Royal Manchester Children’s Hospital, part of Manchester University NHS Foundation Trust, led by David Keene; and the Department of Pediatric Urology and Evelina London Children’s Hospital, part of Guy’s and St Thomas’ NHS Foundation Trust, led by Mr Pankaj Mishra, make up the remaining sites participating in the clinical trial.
This study was reviewed and approved by the West of Scotland Research Ethics Committee, the Medicines and Healthcare products Regulatory Agency (MHRA) and the Health Research Authority (HRA). A total of 150 patients will participate in the study, which will include thorough clinical follow-up of their surgical outcomes, recovery, and clinical outcomes for up to 1 year after surgery.
“We are delighted to be launching the world’s first pediatric trial using Versius in the UK,” said Mark Slack, co-founder and chief medical officer of CMR Surgical. “The small, modular design of the Versius and the small diameter of the tool provide the opportunity to perform robotic-assisted surgery for the treatment of pediatric patients. We are committed to responsibly introducing Versius into new specialties and look forward to working with our partners to conduct this clinical trial to ensure the safety and efficacy of Versius in pediatrics.”
Versius has now been used in more than 23,000 surgical cases, with more than 130 different surgical procedures performed in seven surgical disciplines. The system is routinely used in urology, general surgery, gynecology, colorectal surgery and thoracic surgery. Versius is also being used in an ongoing clinical trial evaluating the suitability of the system for use in transoral robotic surgery (TORS) at Liverpool University Hospitals NHS Foundation Trust (LUHFT) Aintree University Hospital.